
īulk caesium iodide crystals have the cubic CsCl crystal structure, but the structure type of nanometer-thin CsI films depends on the substrate material – it is CsCl for mica and NaCl for LiF, NaBr and NaCl substrates. Synthesis and structure Monatomic caesium halide wires grown inside double-wall carbon nanotubes. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Radioactive cesium does have a chance of entering plants by falling on leaves.Īnimals that are exposed to very high doses of cesium show changes in behaviour, such as increased or decreased activity.Caesium iodide or cesium iodide ( chemical formula CsI) is the ionic compound of caesium and iodine. It remains within the top layers of soils as it strongly bonds to soil particles and as a result it is not readily available for uptake through plant roots. In soils, however, cesium does not rinse out into the groundwater. In water and soils most cesium compounds are very water-soluble. Both radioactive and stable cesium act the same way within the bodies of humans and animals chemically.Ĭesium in air can travel long distances before settling on earth. Non-radioactive cesium can either be destroyed when it enters the environment or react with other compounds into very specific molecules. The radioactive isotopes can only be decreased in concentration through radioactive decay. Radioactive isotopes of cesium may be released into the air by nuclear power plants and during nuclear accidents and nuclear weapons testing. It is also released into the air, water and soil through mining and milling of ores. How serious the effects are depends upon the resistance of individual persons and the duration of exposure and the concentration a person is exposed to.Ĭesium occurs naturally in the environment mainly from erosion and weathering of rocks and minerals. When the exposure lasts a long time people may even lose consciousness.
Due to this, effects such as nausea, vomiting, diarrhoea and bleeding may occur. When contact with radioactive cesium occurs, which is highly unlikely, a person can experience cell damage due to radiation of the cesium particles. It is not very likely that people experience health effects that can be related to cesium itself. People that work in the nuclear power industry may be exposed to higher levels of cesium, but many precautionary measurements can be taken to prevent this. These accidents have not occurred since the Chernobyl disaster in 1986.

The amount of cesium in foods and drinks depends upon the emission of radioactive cesium through nuclear power plants, mainly through accidents. In air the levels of cesium are generally low, but radioactive cesium has been detected at some level in surface water and in many types of foods. Humans may be exposed to cesium by breathing, drinking or eating.

World production of cesium compounds is just 20 tonnes per year, coming mainly from the Bernic lake (Canada) with a little from Zimbabwe and South-West Africa. Few cesium mineral are know, pollucite is the main: they are silicate magmas cooled from granites. Cesium is used in atomic clocks and more recently in ion propulsion systems.Īlthough cesium is much less abundant than the other alkali metals, it is still more common than elements like arsenic, iodine and uranium. The chloride is used in photoelectric cells, in optical instruments, and in increasing the sensitivity of electron tubes. Cesium salts are used to strenght various types of glass. Cesium is sometimes used to remove traces of oxygen from the vacuum tubes and from light bulbs. Cesium nitrate is used to make optical glasses. Cesium metal oxidized rapidly when exposed to the air and can form the dangerous superoxide on its surface.Ĭesium is used in industry as a catalyst promoter, boosting the performance of other metal oxides in the capacity and for the hydrogenation of organic compounds. Cesium reacts with the halogens to form a fluoride, chloride, bromide, and iodide. Cesium hydroxide is a strong base and attacks glass. Cesium reacts explosively with cold water, and reacts with ice at temperatures above -116☌. Cesium, gallium, and mercury are the only three metals that are liquid at or around room temperature. It is the most electropositive and most alkaline element.

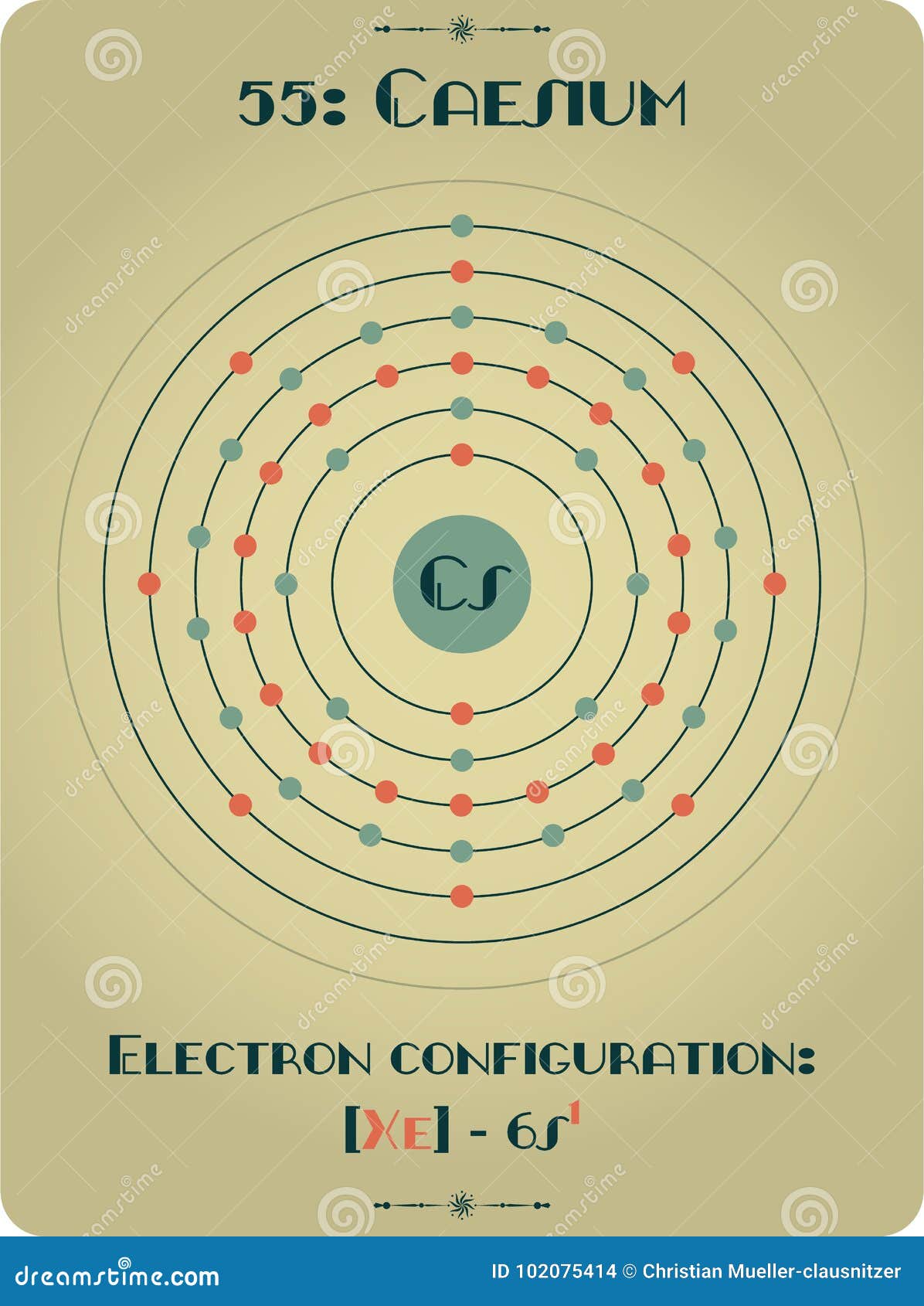
The metal is characterised by a spectrum containing two bright lines in the blue (accounting for its name). Separation and Concentration Purification RequestĬesium - Cs Chemical properties of cesium - Health effects of cesium - Environmental effects of cesium.Plant Inspection & Process Optimalisation.


 0 kommentar(er)
0 kommentar(er)
